- Home
- About Us
- Students
- Academics
-
Faculty
- Electrical Engineering
- Automation
- Computer Science & Engineering
- Electronic Engineering
- Instrument Science and Engineering
- Micro-Nano Electronics
- School of Software
- Academy of Information Technology and Electrical Engineering
- School of Cyber Security
- Electrical and Electronic Experimental Teaching Center
- Center for Advanced Electronic Materials and Devices
- Cooperative Medianet Innovation Center
- Alumni
-
Positions
-
Forum
News
- · Prof. Bin Dai’s team made important progress in using graphene quantum dots to regulate aberrant protein phase separation
- · Guo Gao and co-workers from Shanghai Jiao Tong University achieved significant progress on calcium-ion pre-intercalated hydrated vanadium oxide cathode to facilitate high-performance zinc storage
- · Jiamiao Yang’s team published important innovation on large dynamic range Shack-Hartmann wavefront sensor in Light: Advanced Manufacturing
- · Jiamiao Yang’s team achieved the accurate modeling of the scattering of polarized optical fields
- · Prof. Jingquan Liu’s team made new progress in nanoscale brain-computer interface devices for single neurons
Prof. Bin Dai’s team made important progress in using graphene quantum dots to regulate aberrant protein phase separation
Recently, Prof. Bin Dai’s team, from the School of Electronic Information and Electrical Engineering, along with Prof. Dan Li’s team from the Bio-X Institute at Shanghai Jiao Tong University, and Prof. Liang Wang’s team from the School of Environmental and Chemical Engineering at Shanghai University, have made important progress in using graphene quantum dots (GQDs) to regulate aberrant phase separation of proteins associated with amyotrophic lateral sclerosis (ALS). The research, titled “Halogen Doped Graphene Quantum Dots Modulate TDP-43 Phase Separation and Aggregation in the Nucleus” has been published in the journal Nature Communications.

Figure 1
Background:
In normal physiological conditions, proteins perform multiple important biological functions through liquid-liquid phase separation (LLPS), particularly in the dynamic assembly of membrane-less organelles. However, when the phase separation process becomes aberrant, proteins may shift from a reversible liquid-liquid phase to an irreversible liquid-solid phase, leading to the formation of irreversible amyloid aggregates. Such aggregation is closely linked to various neurodegenerative diseases, including ALS. Consequently, effectively regulating this aberrant phase separation of proteins and thereby inhibiting amyloid fibril formation, is a critical area of current research.
GQDs, known for their ability to cross the blood-brain barrier, excellent biocompatibility, high customizability, and superior optical properties, offer broad potential applications in fields such as chemical biology, biomedical science, and materials science. Our study focuses on the design and synthesis of GQDs that target both the cytoplasm and the nucleus. We explore the effects of these GQDs on the phase separation and amyloid fibril formation of ALS-related pathogenic proteins FUS and TDP-43. This research opens new perspectives and strategies for understanding and treating neurodegenerative diseases.
Research content:
This study reports on design and synthesis of GQDs that can target the nucleus and regulate the phase separation and irreversible aggregation of TDP-43 protein associated with ALS. While many studies focus on TDP-43 aggregation in the cytoplasm, recent findings also document its occurrence within the nucleus, particularly in brain regions compromised by neurodegenerative diseases such as frontotemporal dementia and Alzheimer’s disease. The team synthesized three types of chlorine-modified GQDs using 1,5-diaminonaphthalene as a precursor, which facilitates entry into the nucleus (Fig. 1 a-c).
The results show that these chlorine-modified GQDs can inhibit the formation of TDP-43 granules under oxidative stress or hyperosmotic stimuli in various cell types (Fig. 1 d-e). Additionally, GQDs were found to decrease the formation of nuclear TDP-43 amyloid fibril aggregates and disease-related phosphorylation levels of TDP-43, without impacting overall protein expression (Fig. 1 f-g). Detailed mechanistic studies revealed that GQDs influence TDP-43 phase separation through hydrophobic and electrostatic interactions, directly binding to multiple structural domains of TDP-43, notably the LC domain. Nuclear magnetic resonance spectroscopy analysis further confirms that these binding interactions are independent of specific amino acids (Fig. 1 h). This study underscores the potential of GQDs in regulating nuclear protein LLPS and amyloid fibril aggregation processes.
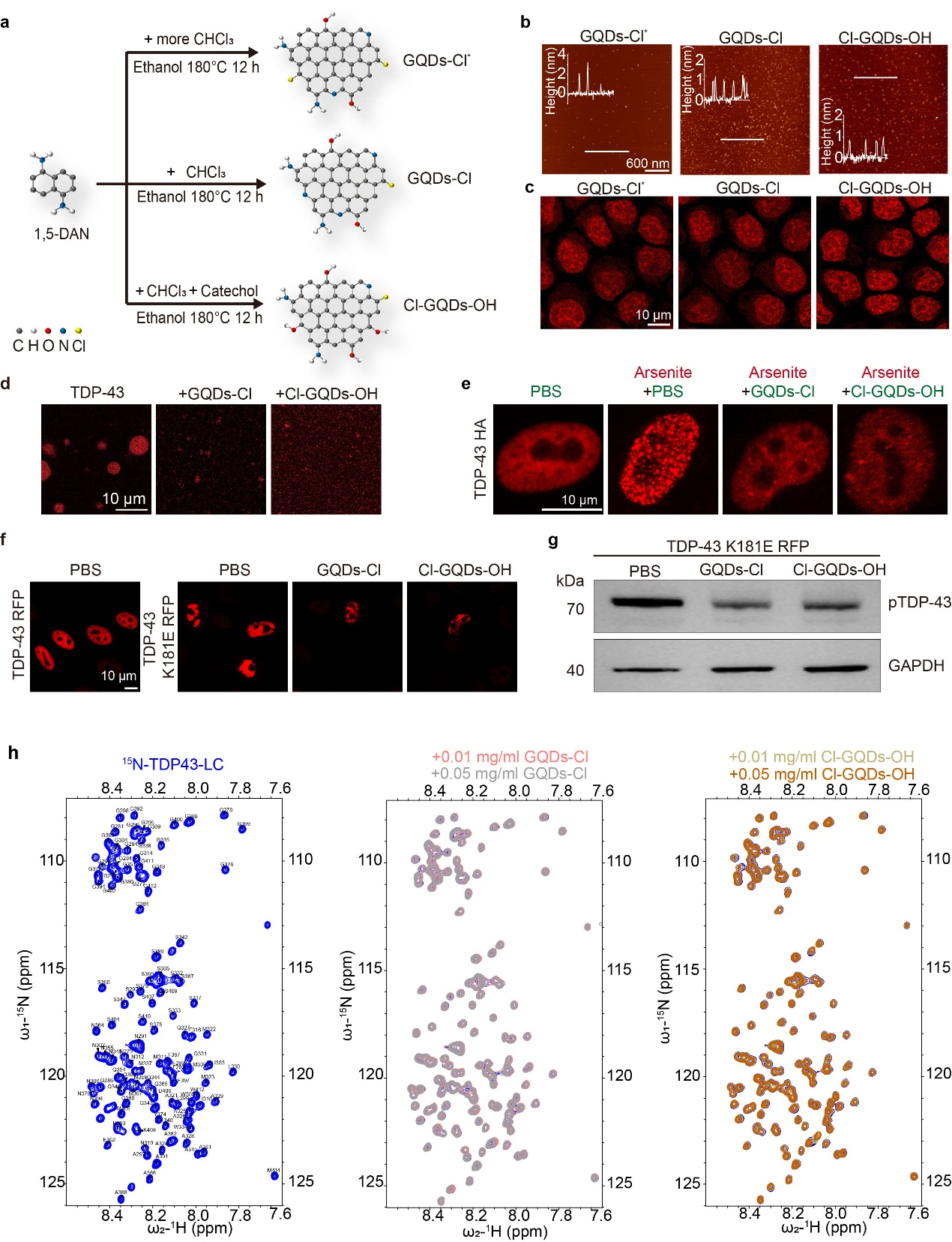
Figure 2. Design, synthesis, and functionality of nucleus-targeting GQDs for regulating phase separation and amyloid fibril aggregation of TDP-43 protein within the nucleus
In 2023, Prof. Bin Dai’s team, along with Prof. Dan Li’s team and Prof. Liang Wang’s team, discovered that GQDs can target the cytoplasm to regulate the phase separation and irreversible aggregation of FUS protein in ALS (ACS Nano 2023, 17, 11, 10129–10141). The teams designed and synthesized three types of GQDs with different surface functional groups, which not only had uniform particle sizes but were also capable of entering the cytoplasm (Fig. 2 a-c). Experimental results showed that these three types of GQDs could co-localize with FUS droplets and significantly inhibit the LLPS of FUS protein (Fig. 2 d). Additionally, the GQDs were able to stabilize the droplet state formed by FUS protein and effectively inhibit the formation of amyloid fibrils within the FUS droplets (Fig. 2 e). GQDs also demonstrated the ability to inhibit the formation of amyloid fibrils by FUS-LC and could dissolve pre-formed FUS-LC fibrils (Fig. 2 f). Further investigation into the molecular mechanisms of interaction between GQDs and FUS protein revealed that all three types of GQDs could bind to both FUS-LC monomers and FUS-LC amyloid fibrils with different binding strengths. Therefore, by finely designing the surface functional groups of GQDs, their regulatory effects on protein phase separation and fibrillization can be controlled. This discovery not only reveals the potential applications of GQDs in regulating protein phase separation and fibrillization but also lays the foundation for the design of novel nanomaterials to control protein phase separation behaviors.
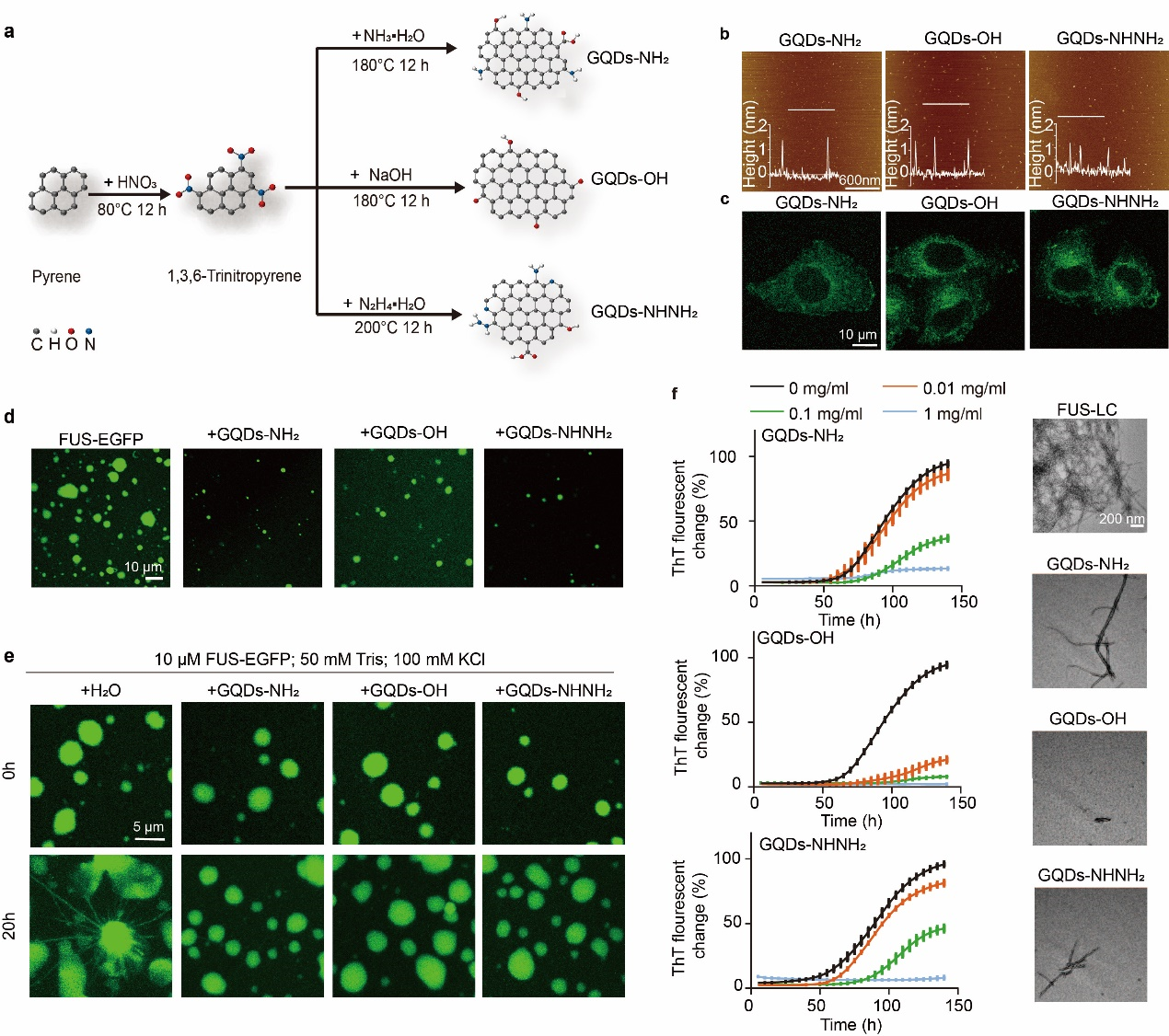
Figure 3. Design and synthesis of cytoplasm-targeting GQDs and their role in regulating the phase separation and amyloid fibril aggregation of FUS protein
These two studies collectively demonstrate the significant capabilities of GQDs in regulating the assembly and disassembly of biomolecular condensates of ALS pathogenic proteins FUS and TDP-43, in both the nucleus and the cytoplasm, as well as their role in inhibiting or reversing amyloid fibrillization (Fig. 3). An in-depth exploration of the mechanisms of interaction between GQDs and these proteins not only reveals the potential applications of GQDs in the treatment of ALS but also deepens the understanding of the pathogenic mechanisms of ALS, particularly the regulatory mechanisms of abnormal protein phase separation, thereby laying a solid foundation for the development of new therapeutic strategies and perspectives.
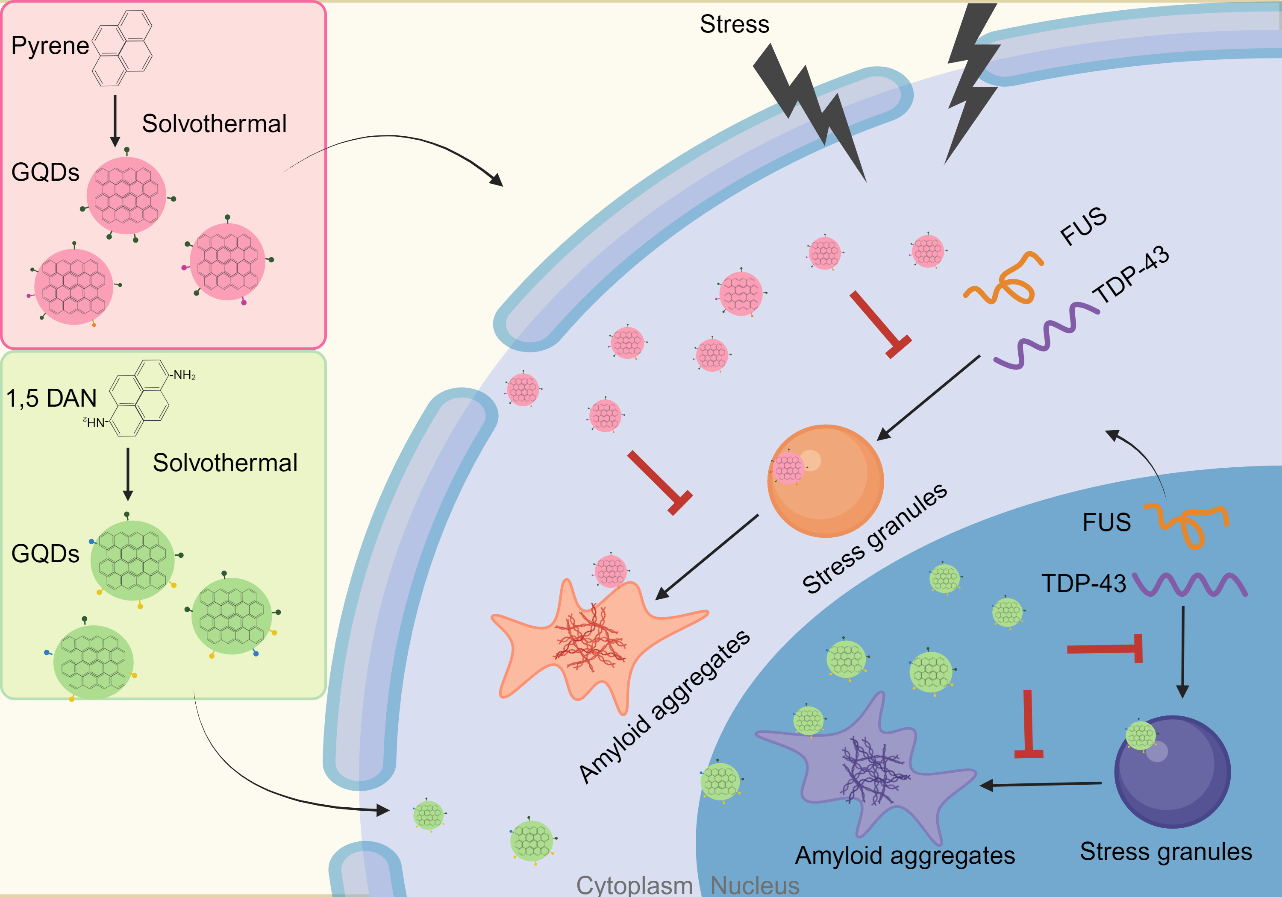
Figure 4. Schematic diagram of GQDs regulating protein LLPS and fibrillization
Prof. Bin Dai from the School of Electronic Information and Electrical Engineering, Prof. Dan Li from the Bio-X Institutes of Shanghai Jiao Tong University, and Prof. Liang Wang from Shanghai University are the corresponding authors. Ph. D. Hong Zhang is the first author. Researcher Cong Liu from the Interdisciplinary Research Center on Biology and Chemistry of Chinese Academy of Sciences and other co-authors also made significant contributions. The School of Electronic Information and Electrical Engineering at Shanghai Jiao Tong University is the primary affiliation. This work was supported by the National Natural Science Foundation of China, the Shanghai Pujiang Program, among others.
Paper link: https://www.nature.com/articles/s41467-024-47167-x
-
Students
-
Faculty/Staff
-
Alumni
-
Vistors
-
Quick Links
