- Home
- About Us
- Students
- Academics
-
Faculty
- Electrical Engineering
- Automation
- Computer Science & Engineering
- Electronic Engineering
- Instrument Science and Engineering
- Micro-Nano Electronics
- School of Software
- Academy of Information Technology and Electrical Engineering
- School of Cyber Security
- Electrical and Electronic Experimental Teaching Center
- Center for Advanced Electronic Materials and Devices
- Cooperative Medianet Innovation Center
- Alumni
-
Positions
-
Forum
News
- · Shanghai Jiao Tong University professors Lian Yong and Wang Guoxing's team have made remarkable progress in the field of high-efficiency pulse neural network accelerator chips.
- · AI + Urban Science research by AI Institute was selected as cover story in Nature Computational Science!
- · The first time in Asia! IPADS's Microkernel Operating System Research Wins the Best Paper Award at SOSP 2023
- · Delegation from the Institution of Engineering and Technology Visits the School of Electronic Information and Electrical Engineering for Journal Collaboration
- · Associate professor Liangjun Lu and research fellow Jiangbing Du from Shanghai Jiao Tong University made important advancements on large capacity and low power consumption data transmission
Prof. Jingquan Liu's team made important progress on implantable MEMS devices for atrial fibrillation
Recently, Prof. Jingquan Liu’s team from the Department of Micro/Nano Electronics, together with Shanghai Chest Hospital, have made important progress in the field of pulsed field ablation with implantable microelectromechanical system (MEMS) devices. The research entitled “A high-precision, low-voltage pulsed field ablation device with capability of minimally invasive surgery” has been published in the journal Advanced Functional Materials (IF=19.924).

Background
Atrial fibrillation is the most common cardiac arrhythmia, occurring in 1% of people over the age of 60 and causing a variety of symptoms including rapid or chaotic heartbeat, fatigue and chest pain, and an increased risk of stroke. Catheter ablation is an effective treatment for patients with symptomatic, drug-refractory atrial fibrillation. Traditional thermal ablation may be associated with negative events such as esophageal injury, phrenic nerve injury, and pulmonary vein stenosis. In contrast, pulse field ablation uses electrical pulses to cause non-thermal irreversible electroporation and induce target cell death without changing the overall structure of the tissue or affecting other cell types. Pulsed field ablation has been used to treat cancer by killing tumor cells for nearly a decade, but the technique has only recently been applied to the cardiovascular field.
However, the current electrodes in pulsed field ablation studies are characterized by their large size and lack of ablation precision, which often leads to "overkill" during myocardial conduction block. Given the natural variability of cardiac anatomy, current electrodes cannot create perfect lesions without damaging non-target areas, especially for complex structures in the heart such as trabecular tissue. At the same time, large electrodes must apply electrical pulses with amplitudes of up to several thousand volts, which may cause unknown complications.
Research content
This study reports materials and designs of a catheter-integrated microelectrode and sensors that could be used for high-precision and low-voltage pulsed field ablation through minimally invasive operation on a large animal model. The device with a new electrode configuration supports point-by-point ablation with a width of 3.8 mm (~1/10 that of a typical ablation electrode) for individual lesions at the voltage of 300 V (an order of magnitude reduction compared to the current state-of-the-art). This catheter-integrated device will enhance the efficiency and safety of pulsed field ablation, especially for complex cardiac structures, thus facilitating its move to the clinic.
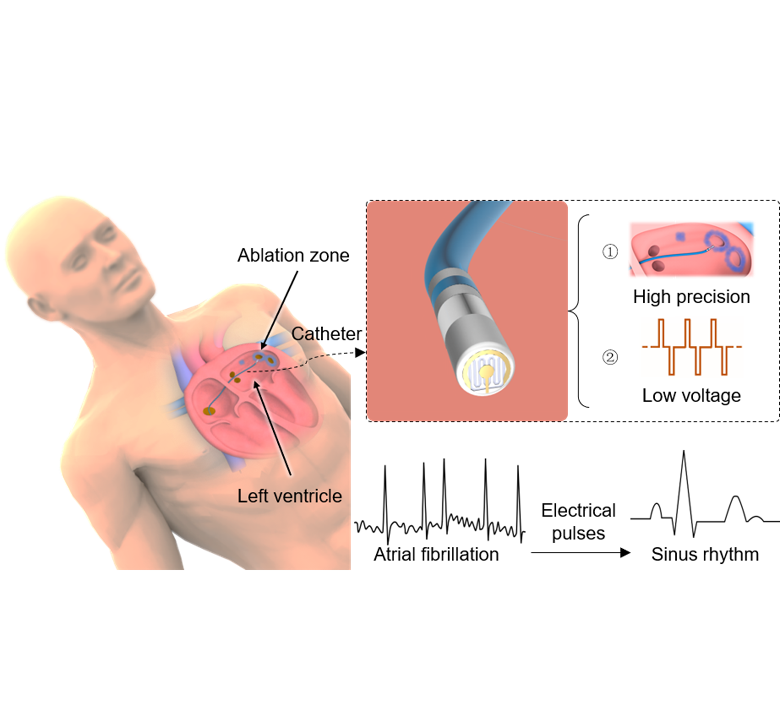
The ablation strategy for the treatment of atrial fibrillation
By analyzing the temperature and electric field distributions in the ablation area, it is essential to predict and optimize the results of clinical procedures. The electrodes are used to apply electrical pulses or radiofrequency signals, and temperature sensors detect the temperature of the ablation area. Through finite element simulations and experimental comparisons, it was determined that cell death during pulsed field ablation is caused by a high electric field and not by an increase in temperature as in RF ablation. In clinical practice, determining the electric field threshold is critical for predicting the depth and magnitude of ablation outcomes. However, most of the current studies stop at the attempt at different ablation parameters for pulsed field ablation and lack a method to quantitatively assess the relationship between ablation parameters and effects. This study proposes a method to determine the electric field threshold for pulsed field ablation through ablation experiments and finite element simulations, which can quantify the results of pulsed field ablation and provide empirical data for electrophysiologists and operators in clinical.
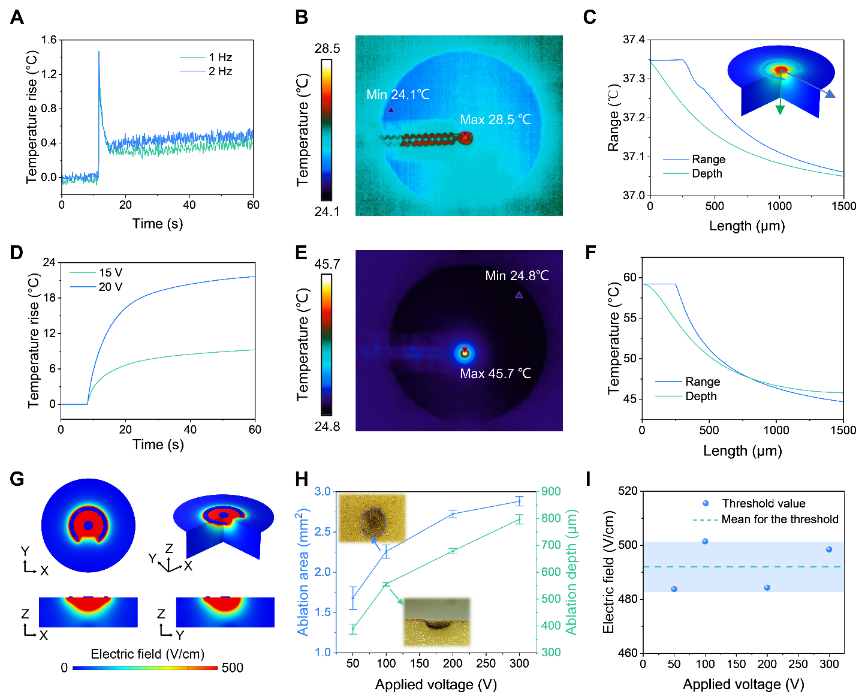
Non-thermal property and the ablation threshold
The integration of electrodes and catheters allows for better operational flexibility in the ablation of cardiac tissue. The study began with a demonstration of low-voltage ablation in rats and immunofluorescence staining of rat heart sections to demonstrate the results at the cellular level. The use of a rat heart model combined with histological examination allows for easy assessment of ablation effects. However, there are inherent differences between rodents and humans, such as histology, anatomy, and structure, that require large animal models that accurately approximate human physiology (e.g., Beagle) to conduct the study. This study used advanced medical equipment to successfully perform endocardial ablation by implanting a MEMS device integrated on a catheter. Before the ablation experiment, an intracardiac echocardiographic catheter was used to construct a three-dimensional model of the heart. A high-density calibrated catheter was then used to measure the potential at the tissue surface. Following a series of electrical pulses, an ablation zone consisting of several consecutive ablation sites was created, and voltage scaling maps showed that the catheter-integrated device can create a continuous effective lesion through point-by-point pulsed field ablation.
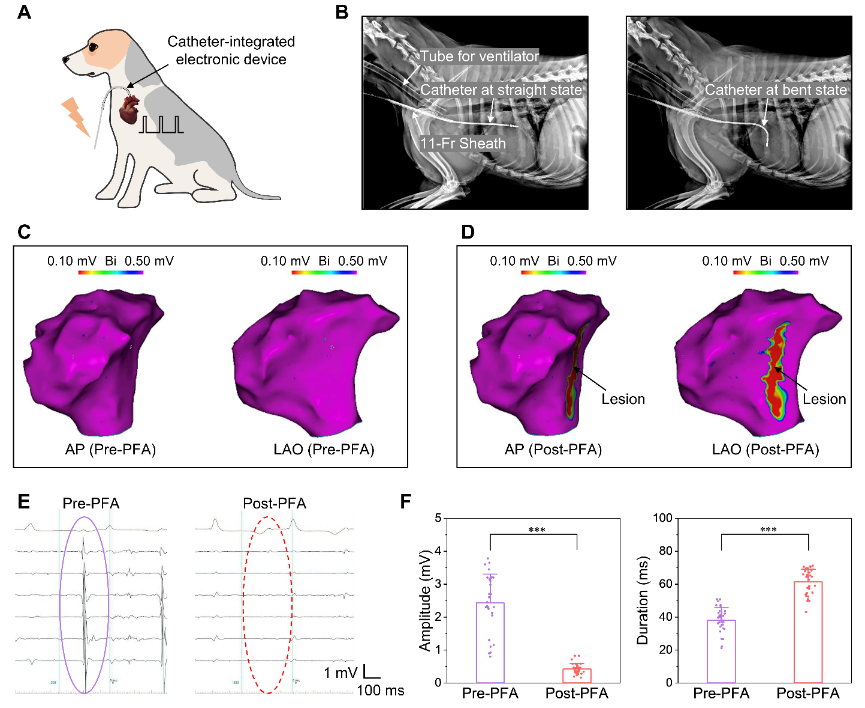
Minimally invasive procedure on the endocardium of beagles
This research is aimed at clinically important diseases and provides new solutions to overcome the shortcomings of conventional medical devices by implementing micro-sensors and actuators through MEMS technologies. In the future, before applying such devices to patients, more preclinical evaluations are needed to determine the performance for long-term follow-up (e.g., one year) and the optimal treatment parameters for pulsed field ablation.
About the paper
Ph.D. candidate Mengfei Xu from the School of Electronic Information and Electrical Engineering and Ph.D. Ziliang Song from Shanghai Chest Hospital are the first authors of this work. Professor Jingquan Liu and Associate Professor Mu Qin are the corresponding authors of this paper. This work was supported by the National Key Research and Development Program, the National Natural Science Foundation, and so on.
Jingquan Liu's team has been working on wearable/implantable flexible electronics, brain-computer interface, intelligent microsensors for extreme environments, and MEMS technology (Sci. Adv. 2021, Adv. Mater. 2021, Small 2019, Microsyst. Nanoeng. 2022 and Biosens. Bioelectron. 2020, etc.), and to promote the clinical translation.
Paper link: https://doi.org/10.1002/adfm.202302041
Team website: http://mems.sjtu.edu.cn/
-
Students
-
Faculty/Staff
-
Alumni
-
Vistors
-
Quick Links
