- Home
- About Us
- Students
- Academics
-
Faculty
- Electrical Engineering
- Automation
- Computer Science & Engineering
- Electronic Engineering
- Instrument Science and Engineering
- Micro-Nano Electronics
- School of Software
- Academy of Information Technology and Electrical Engineering
- School of Cyber Security
- Electrical and Electronic Experimental Teaching Center
- Center for Advanced Electronic Materials and Devices
- Cooperative Medianet Innovation Center
- Alumni
-
Positions
-
Forum
News
- · Bin Dai's Team Unveils the Assembly Mechanism of β-Lactoglobulin Fibrils, Providing New Insights for the Development of Functional Nanomaterials
- · Mingyi Chen’s research group has made important progress in the field of analog-to-digital converter chips for brain-computer interface
- · Progress in the Development of Semiconductor Nanomaterials to Activate Pyroptosis for Cancer Therapy
- · Jiamiao Yang’s team achieved the high precision optoelectronic reservoir computing based on complex-value encoding
- · Significant Advancements in Resonator-Enhanced Quantum Sensing Achieved by Zenguihua's Team at the School of Sensing Science and Engineering
Bin Dai's Team Unveils the Assembly Mechanism of β-Lactoglobulin Fibrils, Providing New Insights for the Development of Functional Nanomaterials
Recently, Prof. Bin Dai's team, from the School of Electronic Information and Electrical Engineering and the School of Perceptual Science and Engineering, has made significant progress in understanding the assembly mechanism and polymorphism of β-lactoglobulin (β-LG) fibrils. Utilizing cryo-electron microscopy, the team revealed the conserved core structure of β-LG fibrils and their diverse aggregation morphologies, providing new theoretical insights for the precise regulation of β-lactoglobulin-based functional materials. The research, titled "β-Lactoglobulin Forms a Conserved Fibril Core That Assembles into Diverse Fibril Polymorphs," have been published in the prestigious international journal Nano Letters.

Background
Proteins naturally self-assemble into nanoscale fibril structures, which have been widely utilized in the fields of food science, biomedicine, and materials science. β-Lactoglobulin (β-LG), one of the major components of whey protein, can self-assemble into fibrils under acidic conditions (pH 2) and high temperatures (90°C). These fibrils not only influence the rheological properties of food but also exhibit great potential in applications such as drug delivery, biosensing, and smart materials due to their highly stable and tunable nanostructures.
Despite extensive studies on the formation of β-LG fibrils, the molecular-level assembly mechanisms and the principles governing their structural polymorphism remain poorly understood. This gap has hindered the rational design and optimization of β-LG-based functional materials. Prof. Bin Dai's team utilized high-resolution cryo-electron microscopy (cryo-EM) to resolve the core structure of β-LG fibrils, unveiling the mechanisms underlying their polymorphic assembly. These findings provide a fundamental theoretical basis for the design of β-LG fibril-based nanomaterials.
Research content
Prof. Bin Dai's team employed cryo-EM to determine the high-resolution structure of β-LG fibrils, revealing that their core consists of two protofibrils, each formed by residues 1-32 of the β-LG sequence. Further analysis demonstrated that individual protofibrils can assemble into four distinct fibril polymorphs through different interfacial interactions (Figure 1). The research found that the stability of the core structure is primarily maintained by hydrophobic interactions. Key residues, including Leu10, Ile12, Val15, Trp19, and Leu22, interact to form a stable U-shaped folding pattern, ensuring the robustness of the fibril core.
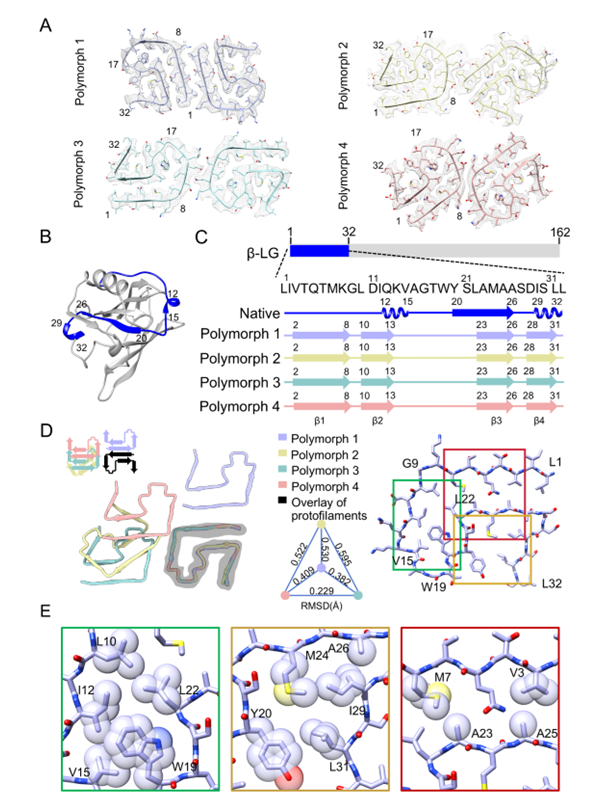
Figure 1. Polymorphic structures of β-LG fibrils and intramolecular interactions within protofibrils.
Different interactions at interfaces, including hydrophilic interactions, hydrogen bonding, and electrostatic forces, dictate the formation of the four distinct β-LG fibril polymorphs (Figure 2). These structural characteristics endow β-LG fibrils with highly tunable mechanical properties and chemical stability, offering a diverse structural platform for the development of protein-based functional materials.
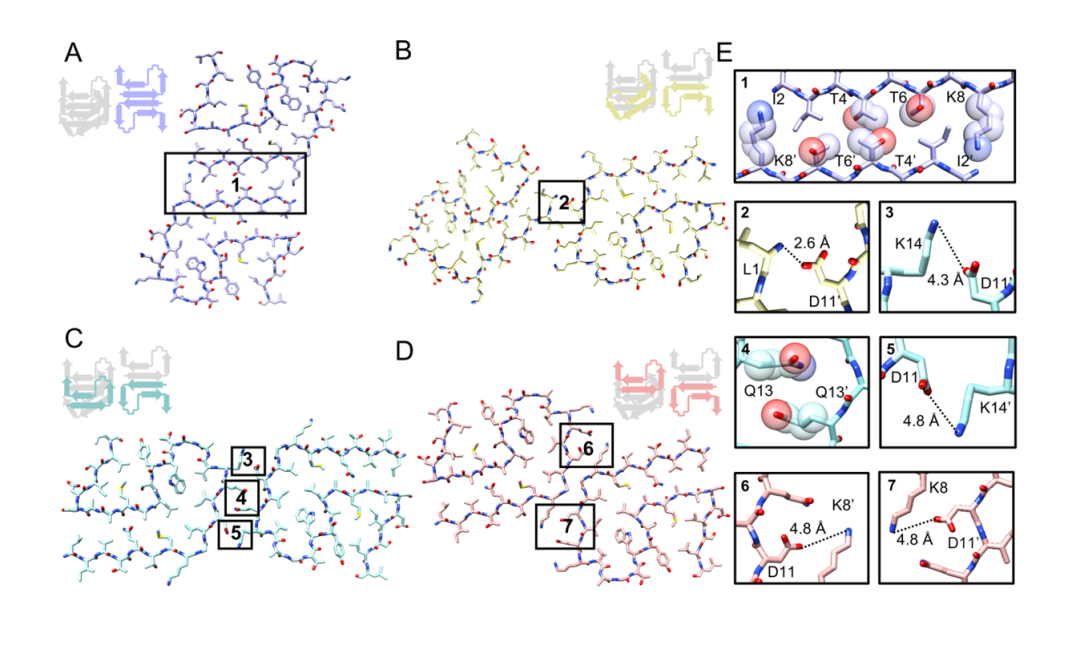
Figure 2. Analysis of interfacial features in four fibril polymorphs.
This study not only deepens the understanding of the molecular assembly mechanism of β-LG fibrils but also provides new insights for the design of β-LG fibril-based materials, including food thickeners, biosensors, functional coatings, and drug delivery systems.
Research Team
This research was co-authored by Yongyi Xu and Danni Li, doctoral students from the School of Electronic Information and Electrical Engineering, and the School of Perceptual Science and Engineering at Shanghai Jiao Tong University, as joint first authors. Associate Professor Bin Dai from the School of Electronic Information and Electrical Engineering, and the School of Perceptual Science and Engineering at Shanghai Jiao Tong University served as the corresponding author. Researcher Cong Liu from the Center for Excellence in Molecular Cell Science, Chinese Academy of Sciences, Professor Dan Li from Shanghai Jiao Tong University and other contributors, also made significant contributions to this research. Shanghai Jiao Tong University is the primary affiliation for this study. This work was supported by grants from the National Natural Science Foundation of China, the Science and Technology Commission of Shanghai Municipality, and other funding sources.
Paper link: https://pubs.acs.org/doi/10.1021/acs.nanolett.5c00137
-
Students
-
Faculty/Staff
-
Alumni
-
Vistors
-
Quick Links
