- Home
- About Us
- Students
- Academics
-
Faculty
- Electrical Engineering
- Automation
- Computer Science & Engineering
- Electronic Engineering
- Instrument Science and Engineering
- Micro-Nano Electronics
- School of Software
- Academy of Information Technology and Electrical Engineering
- School of Cyber Security
- Electrical and Electronic Experimental Teaching Center
- Center for Advanced Electronic Materials and Devices
- Cooperative Medianet Innovation Center
- Alumni
-
Positions
-
Forum
News
- · Shanghai Jiao Tong University professors Lian Yong and Wang Guoxing's team have made remarkable progress in the field of high-efficiency pulse neural network accelerator chips.
- · AI + Urban Science research by AI Institute was selected as cover story in Nature Computational Science!
- · The first time in Asia! IPADS's Microkernel Operating System Research Wins the Best Paper Award at SOSP 2023
- · Delegation from the Institution of Engineering and Technology Visits the School of Electronic Information and Electrical Engineering for Journal Collaboration
- · Associate professor Liangjun Lu and research fellow Jiangbing Du from Shanghai Jiao Tong University made important advancements on large capacity and low power consumption data transmission
Prof. Jingquan Liu and Prof. Yuezeng Su's teams made important progress on implantable MEMS devices for inflammation inhibition during tendon rupture
Recently, Prof. Jingquan Liu and Prof. Yuezeng Su's teams, from the School of electronic information and electrical engineering, together with Shanghai Sixth People's Hospital Affiliated to Shanghai Jiao Tong University School of Medicine, have made important progress in the field of inflammation inhibition with implantable microelectromechanical system (MEMS) devices. The research entitled “Stimulation of Sympathetic Nerve Using Ultraflexible Cuff Electrodes Inhibits Inflammation Caused by Tendon Rupture” has been published in the journal Advanced Functional Materials (IF=19.0).
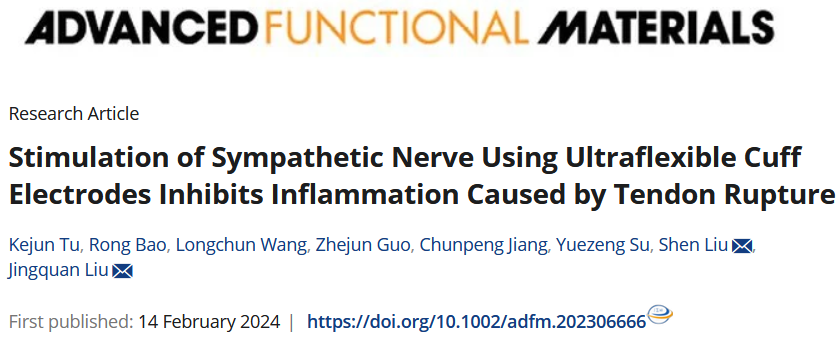
1. Background
Tendon rupture is a severe and debilitating injury among individuals, especially athletes, whose careers will end if they suffer from it. One critical biological change caused by this injury is the inflammatory factors accumulation, leading to peritendinous adhesion, thus hindering the tendon recovery. Therefore, an effective way to treat tendon injury is to inhibit the aggregation of surrounding inflammatory factors.
Pharmacological treatments, such as nonsteroidal anti-inflammatory drugs, are commonly used to suppress peritendinous inflammation by reducing acute inflammatory cytokines; however, they are inefficient and have cardiovascular and genitourinary side effects. Bioengineering therapy is an increasingly popular treatment; however, it still has problems with tendon re-rupture, infection, and severe foreign body inflammatory reactions. Recently, electrical stimulation (ES) of nerves has emerged as a promising therapeutic approach with the advantages of efficacy and specificity, which was widely applied in deep brain stimulation for the treatment of epilepsy and depression, visual cortex stimulation for the vision recovery, and cochlear stimulation for the hearing restoration, etc. However, rarely has an article reported the ES on specific nerve for the treatment of injured tendons. Here, Su and Liu et al probed the relationship between the sympathetic ES and inflammation level during tendon rupture through flexible MEMS electronics, and preliminarily validated the underlying biological mechanisms.
2. Research content
This study reports materials and designs of an ultraflexible cuff electrode with paired microelectrodes sites that could be conformally wrapped around sympathetic nerve (a maximum diameter of about 0.2 mm) for efficient electrical stimulation and recording through minimally invasive operation on rodent model. Different from commercial electrode, the thickness of fabricated ultraflexible cuff electrode is only 3.3 um, which can be easily wrapped around the ultrathin nerves (<0.2 mm) just with the capillary force and cause less damage to the surrounding tissues. Furthermore, the microelectrode sits are coated with platinum nanomaterials to improve the electrochemical performance and durability, thus facilitating the move to clinical long-term application.
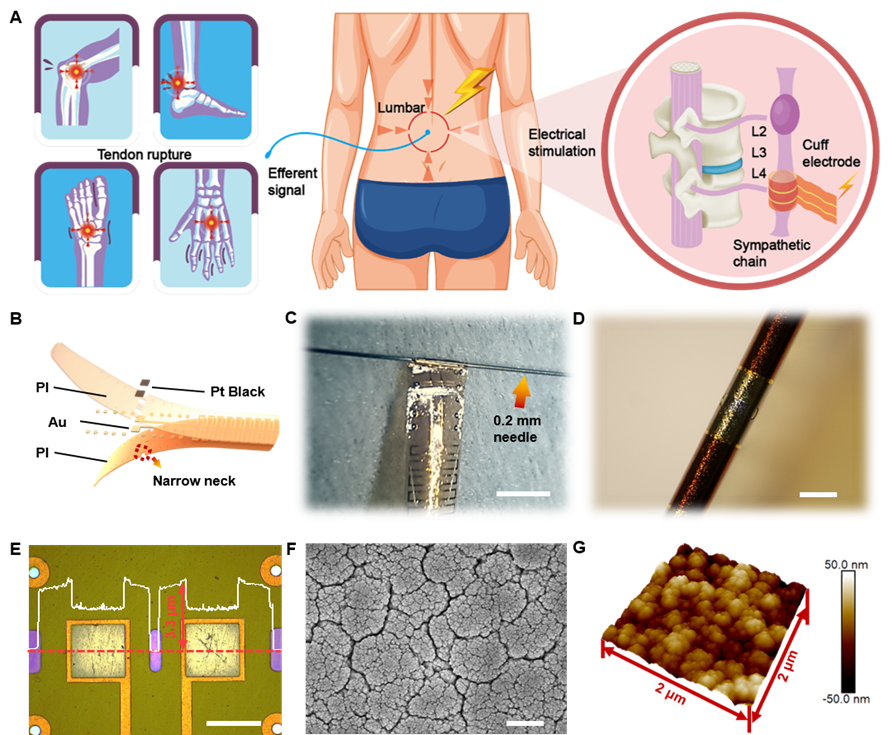
Sympathetic nerve stimulation based on ultraflexible cuff electrodes
Verifying the current pathway between the sympathetic nerve and the tendon is critical for further probing inflammation modulation by ES. Su and Liu et al implanted an ultraflexible cuff electrode into the sympathetic chain ganglia (SchG) of the mouse through the retroperitoneal space and performed in vivo stimulation. Another ultraflexible cuff electrode was wrapped around the tendon to record the evoked potential. The results showed that the corresponding evoked compound nerve action potentials were apparent and exhibited the same frequency as the stimulus waveforms, and peak potentials increased as current intensity rose. These results confirmed the directionality and effectiveness of ES, providing empirical data for the ES on sympathetic nervous system to treat tendon rupture.
.
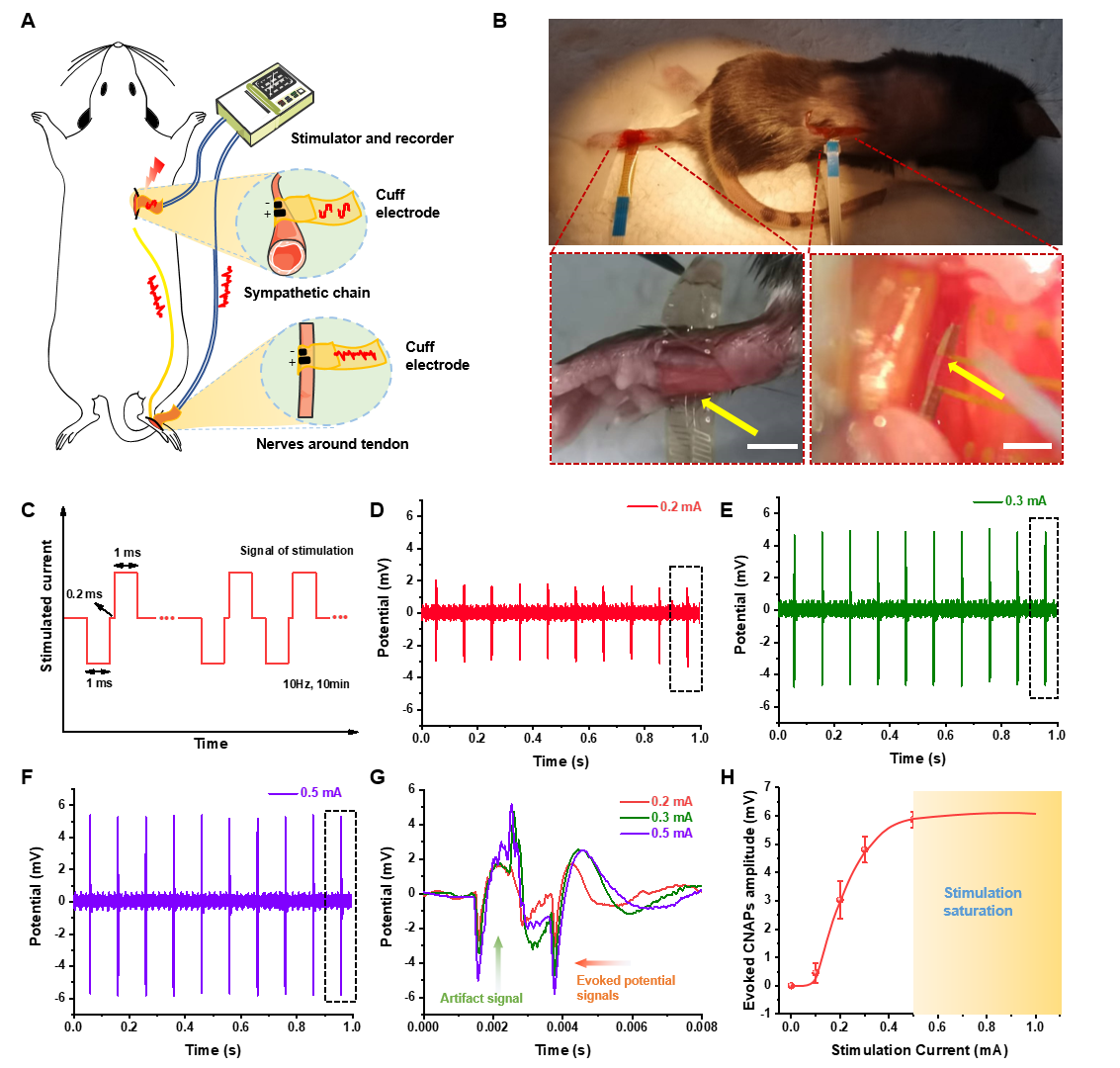
Simultaneous stimulation of sympathetic chain and recording of nerve endings around tendons
The following immunofluorescence staining verify that sympathetic nerves exist in both the SchG and hind paws and reconfirm that sympathetic ES could be transmitted from the SchG to tendons. To determine the optimal ES current, current intensities ranging from 0.03–1 mA were delivered and the inflammation level around the injured tendon were tested. The results showed that current intensity up to 0.2 mA could significantly decrease the concentration of inflammatory factors (TNF-α, IL-1β and IL-6). As the lower current causes less damage, the optimal current of effective ES was determined as 0.2 mA. Meanwhile, the neurotransmitter of the sympathetic nervous system (norepinephrine) was increased by almost an order of magnitude in the ruptured tendon, together with more neural activity was recorded after ES. Further mechanism explorations suggested that ES caused the release of NE from SchG, and NE decreased inflammation after tendon injury through β2 adrenergic receptor (Adrb2).
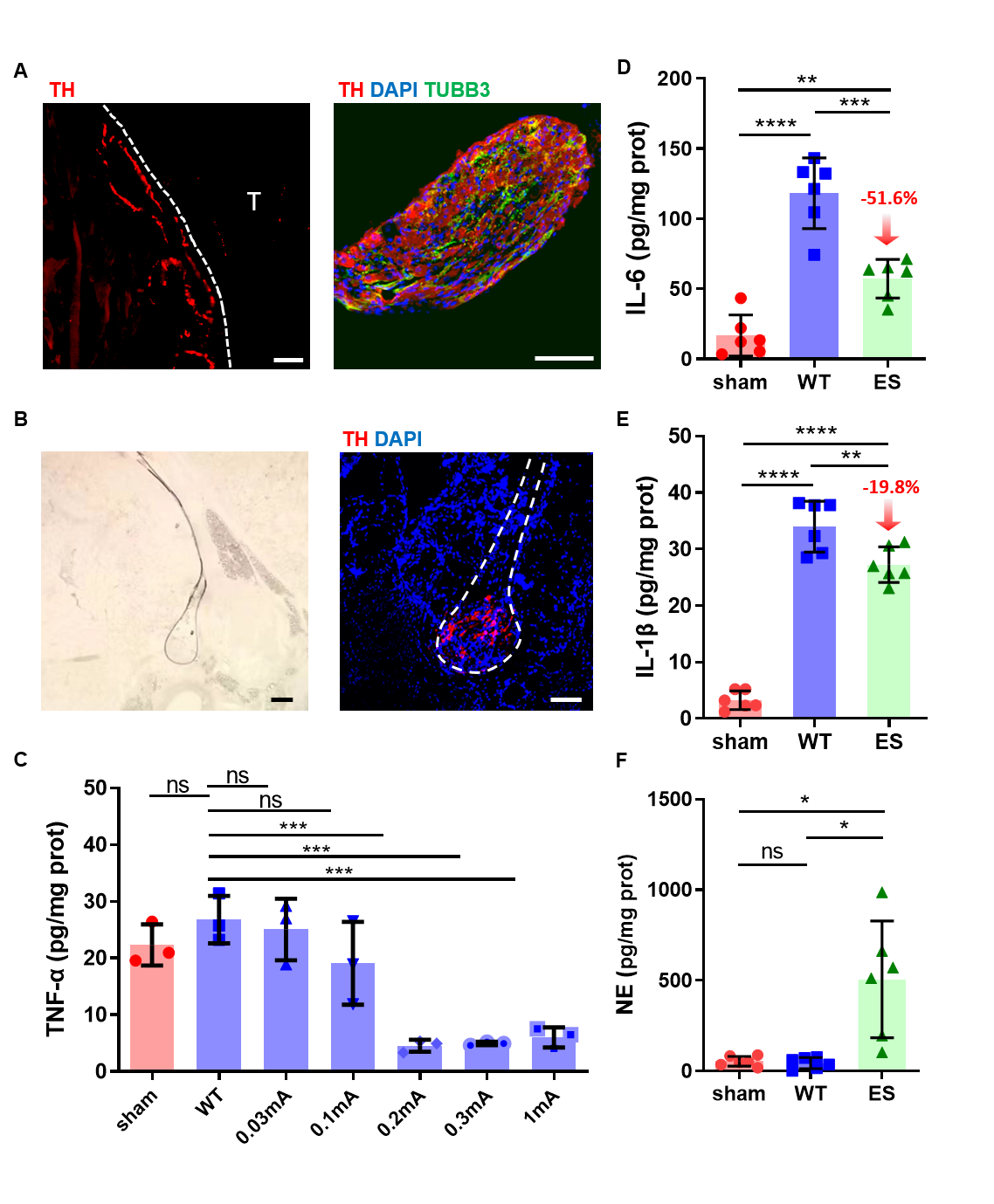
ES on the SchG inhibits inflammation after tendon injury
This research is aimed at clinically important diseases and provides new solutions to overcome the shortcomings of conventional medical devices by implementing micro-recorders and stimulators through integrated circuit technologies. To the best of the knowledge, this is the first to verify that the ES of the sympathetic nerve can inhibit inflammation around the tendon, bringing new inspiration for the tendon rupture treatment. Future work will include an in-depth discussion of the biological mechanism underlying sympathetic ES, long-term in vivo evaluation of electrode's effectiveness in inhibiting inflammation, as well as systematic integration of electrodes, stimulators, and recorders with wireless operation, further promoting the conversion of research into clinical practice.
3. About the paper
Ph.D. Kejun Tu from the School of Electronic Information and Electrical Engineering and Ph.D. Rong Bao from Shanghai Sixth People's Hospital are the first authors of this work. Professor Jingquan Liu and Professor Shen Liu are the corresponding authors of this paper. This work was supported by the National Key Research and Development Program, the National Natural Science Foundation, and so on.
Jingquan Liu's team has been working on wearable/implantable flexible electronics, brain-computer interface, and MEMS technology (Sci. Adv. 2021, Adv. Mater. 2021, Adv. Funct. Mater. 2023, Small 2019, Microsyst. Nanoeng. 2022 and Biosens. Bioelectron. 2020, etc.), and to promote the clinical translation.
Paper link: https://doi.org/10.1002/adfm.202306666
Team website: http://mems.sjtu.edu.cn/
-
Students
-
Faculty/Staff
-
Alumni
-
Vistors
-
Quick Links
